Draw The Major Elimination Product Formed In The Reaction
Draw The Major Elimination Product Formed In The Reaction - There are 3 steps to solve this one. Web in many cases one major product will be formed, the most stable alkene. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Web the major product of an elimination reaction tends to be the more substituted alkene. Web draw the major elimination product formed in the following reaction. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then.
draw the major elimination product formed in the reaction redandyellowvans
Web the major product of an elimination reaction tends to be the more substituted alkene. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web in many cases one major product will be formed, the most stable alkene. Web to find the products in a.
Draw the major elimination product formed in the reaction. Select Draw Rings More CH3OK+ DMSO
This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. Web in many cases one major product will be formed, the most stable.
draw the major elimination product formed in the reaction share4uhoshiro
Web draw the major elimination product formed in the following reaction. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Web in many cases one major product will be formed, the most stable alkene. Alkyl halides undergo elimination via two common mechanisms, known as e2.
Solved Draw the major elimination product formed in the
Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Web in many cases one major product will be.
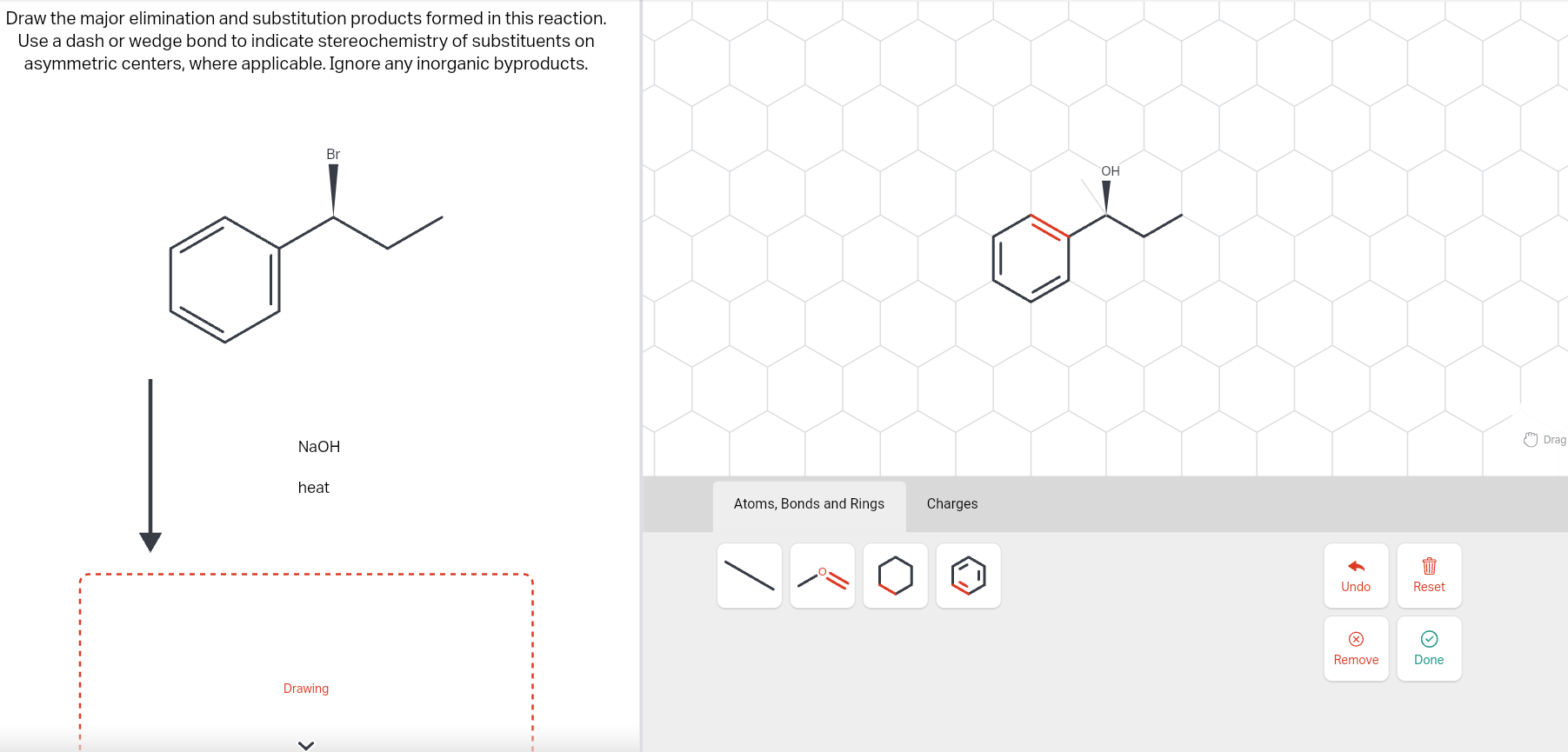
Solved Draw the major elimination and substitution products
In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. Web if two or more structurally distinct groups of adjacent hydrogens are present.
Solved Draw the major elimination and substitution products
Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web the major product of an elimination reaction tends to be the more.
Solved Draw the major elimination and substitution products
Web the major product of an elimination reaction tends to be the more substituted alkene. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric.
Solved Draw the major elimination product formed in the
Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. Web in many cases one major product will be formed, the most stable alkene. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. This is because the transition state leading to.
Solved Draw the major elimination and substitution products
Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. Web draw the major elimination product formed in the following reaction. There are 3 steps to solve this one. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more.
[Solved] draw the major product formed. Draw the major elimination product... Course Hero
Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. Web draw the major elimination product formed in the following reaction. Web the major.
Web draw the major elimination product formed in the following reaction. Web in many cases one major product will be formed, the most stable alkene. Web the major product of an elimination reaction tends to be the more substituted alkene. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. There are 3 steps to solve this one. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions.
Zaitsev’s Rule Is An Empirical Rule Used To Predict The Major Products Of Elimination Reactions.
Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. There are 3 steps to solve this one.
Web In Many Cases One Major Product Will Be Formed, The Most Stable Alkene.
In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. Web draw the major elimination product formed in the following reaction. Web the major product of an elimination reaction tends to be the more substituted alkene.