How Is A Cation Formed
How Is A Cation Formed - Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical. Web learn how cations are formed by the loss of one or more electrons from atoms. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. Web cations are formed by the loss of one or two electrons from an element. See examples of cation formation for alkali. Groups 1 and 2 elements form cations. Explain how neutral atoms ionize to form cations. Determine the charges achieved when.
PPT ChemCatalyst PowerPoint Presentation, free download ID5516040
Web cations are formed by the loss of one or two electrons from an element. Groups 1 and 2 elements form cations. Web learn how cations are formed by the loss of one or more electrons from atoms. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. Web a cation (a positive.
Cation Ap Chemistry, Protons, Anatomy And Physiology, Sodium, Physics, Diagram, Positivity
Explain how neutral atoms ionize to form cations. Groups 1 and 2 elements form cations. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical..
The Difference Between a Cation and an Anion
Groups 1 and 2 elements form cations. Web cations are formed by the loss of one or two electrons from an element. Web learn how cations are formed by the loss of one or more electrons from atoms. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. Web learn how cations and.
Cations and Anions Difference between Cations and Anions
Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. See examples of cation formation for alkali. Groups 1 and 2 elements form cations. Determine the charges achieved when.
Cations vs Anions Difference Between Cations and Anions with Examples
Groups 1 and 2 elements form cations. Web cations are formed by the loss of one or two electrons from an element. See examples of cation formation for alkali. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Determine the charges achieved when.
PPT Ions and Ionic Compound PowerPoint Presentation, free download ID3011004
Explain how neutral atoms ionize to form cations. Determine the charges achieved when. Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical. Web learn how cations are formed by the loss of one or more electrons from atoms. Web cations are formed by the loss of one or two.
Cations and Anions Definitions, Examples, and Differences
Explain how neutral atoms ionize to form cations. Web cations are formed by the loss of one or two electrons from an element. Web learn how cations are formed by the loss of one or more electrons from atoms. Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical. Determine.
Ions Meaning and Examples [in Chemistry] Teachoo Concepts
Web cations are formed by the loss of one or two electrons from an element. See examples of cation formation for alkali. Explain how neutral atoms ionize to form cations. Web learn how cations are formed by the loss of one or more electrons from atoms. Web during the formation of some compounds, atoms gain or lose electrons, and form.
PPT Ionic Bonding PowerPoint Presentation, free download ID2664362
Groups 1 and 2 elements form cations. Web cations are formed by the loss of one or two electrons from an element. Determine the charges achieved when. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web learn how cations and anions are formed.
Naming Ionic Compounds Chemistry Steps
Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical. Web learn how cations are formed by the loss of one or more electrons from atoms. Groups 1 and 2 elements form cations. See examples of cation formation for alkali. Web a cation (a positive ion) forms when a neutral.
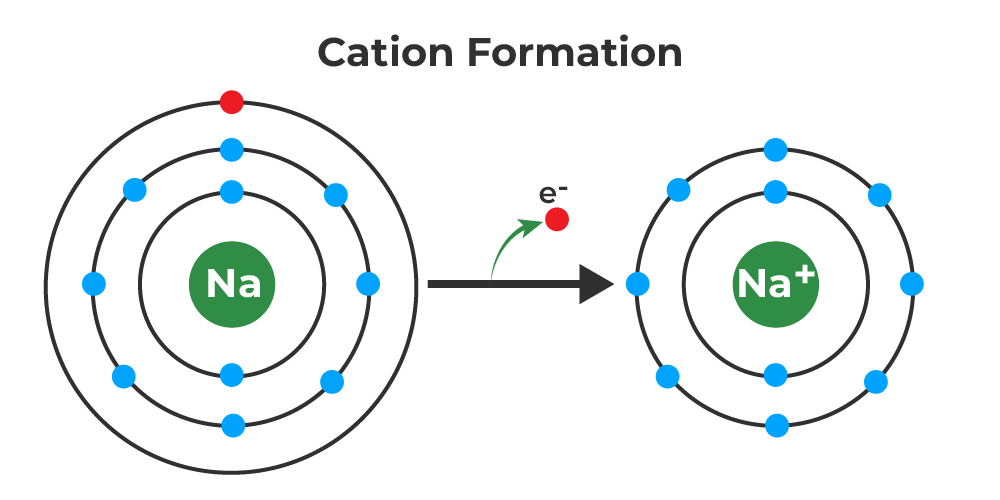
Groups 1 and 2 elements form cations. Web cations are formed by the loss of one or two electrons from an element. Web learn how cations are formed by the loss of one or more electrons from atoms. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Determine the charges achieved when. Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical. Explain how neutral atoms ionize to form cations. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. See examples of cation formation for alkali.
Web During The Formation Of Some Compounds, Atoms Gain Or Lose Electrons, And Form Electrically Charged Particles.
Web learn how cations and anions are formed by losing or gaining electrons, and how they are written in chemical. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Determine the charges achieved when. Web learn how cations are formed by the loss of one or more electrons from atoms.
See Examples Of Cation Formation For Alkali.
Explain how neutral atoms ionize to form cations. Web cations are formed by the loss of one or two electrons from an element. Groups 1 and 2 elements form cations.



/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)



![Ions Meaning and Examples [in Chemistry] Teachoo Concepts](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/eb2b7929-97cd-4d38-9a03-b15e74a5e461/generation-of-cation-01.jpg)

