What Ion Does Aluminum Form
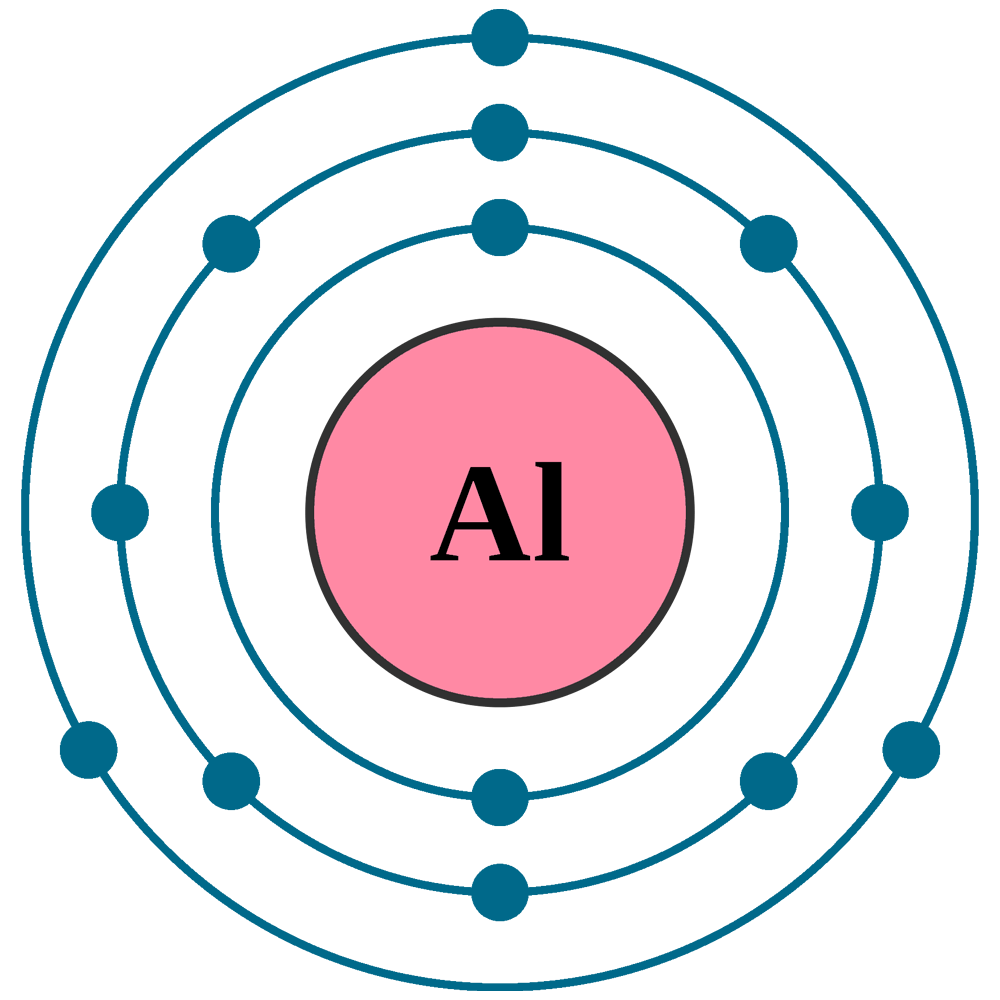
What Ion Does Aluminum Form - Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13. Web an atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence. Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Aluminum is the most abundant. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Web the charge of an aluminum ion is typically 3+. Web 93 rows ionic charge: Web learn how to name positive ions (cations), negative ions (anions), and ionic compounds involving main group elements. Predict which forms an anion, which forms a cation, and the.
PPT 1 Name the ions formed by these elements and classify them as anions or cations
When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Web 93 rows ionic charge: Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Web learn how to name positive ions (cations), negative ions (anions),.
How to Find the Valence Electrons for Aluminum (Al)?
Aluminum is the most abundant. Web an atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence. Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table. Predict which forms an anion, which forms a cation, and the. Web 93 rows ionic charge:
Aluminium Al (Element 13) of Periodic Table Elements FlashCards
Predict which forms an anion, which forms a cation, and the. Web the charge of an aluminum ion is typically 3+. Aluminum is the most abundant. Web learn how to name positive ions (cations), negative ions (anions), and ionic compounds involving main group elements. Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table.
aluminum Uses, Properties, & Compounds Britannica
Aluminum is the most abundant. Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table. Web the charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13. Web an atom of aluminum contains 13 protons and 13.
How many valence electrons does aluminum(Al) have?
Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Aluminum is the most abundant. Predict which forms an anion, which forms a cation, and the. Web 93 rows ionic charge: Web learn how to name positive ions (cations), negative ions (anions), and ionic compounds involving.
Electron arrangements
Aluminum is the most abundant. Web an atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence. Web 93 rows ionic charge: This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13. Web aluminum oxide is relatively unreactive because the small al 3 +.
Aluminum Ion
Web aluminum and carbon react to form an ionic compound. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Aluminum is the most abundant. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13. Web aluminum oxide is relatively unreactive.
Al 3+ Electron Configuration (Aluminum Ion) Electron configuration, Electrons, Configuration
Predict which forms an anion, which forms a cation, and the. Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table. When the atom loses or gains one or more.
Diagram Representation Of The Element Aluminium Stock Vector Image 59012999
Aluminum is the most abundant. Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table. Web the charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13. Web aluminum and carbon react to form an ionic compound.
PPT Al 3+ Aluminum ion PowerPoint Presentation, free download ID2508210
Aluminum is the most abundant. Predict which forms an anion, which forms a cation, and the. Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed)..
Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table. Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Web aluminum and carbon react to form an ionic compound. Aluminum is the most abundant. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13. Web an atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence. Predict which forms an anion, which forms a cation, and the. Web 93 rows ionic charge: Web the charge of an aluminum ion is typically 3+. Web learn how to name positive ions (cations), negative ions (anions), and ionic compounds involving main group elements.
Web Learn How To Name Positive Ions (Cations), Negative Ions (Anions), And Ionic Compounds Involving Main Group Elements.
Web aluminum and carbon react to form an ionic compound. Web an atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence. Web aluminum, chemical element, a lightweight silvery white metal of group 13 of the periodic table. Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in.
Aluminum Is The Most Abundant.
Web 93 rows ionic charge: Web the charge of an aluminum ion is typically 3+. Predict which forms an anion, which forms a cation, and the. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).