Which Pair Of Atoms Forms The Most Polar Bond
Which Pair Of Atoms Forms The Most Polar Bond - In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge between them. Web bonds between two nonmetals are generally covalent; Web a polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the. Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most polar: In other words, a polar bond forms an electric dipole. An oxidant is a substance that can accept the electrons from another reagent. Web polar bonds are intermediate between pure covalent bonds and ionic bonds. Web which pair of atoms forms the most polar bond? Bonding between a metal and a nonmetal is often ionic. Web in a polar bond, one atom has a partial positive electrical charge, while the other atom has a partial negative electrical charge.
Solved Which pair of atoms forms the most polar bond?
In other words, a polar bond forms an electric dipole. Bonding between a metal and a nonmetal is often ionic. Web in a polar bond, one atom has a partial positive electrical charge, while the other atom has a partial negative electrical charge. In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge.
Which Pair Of Atoms Forms The Most Polar Bond
Web a polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the. Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most polar: Web which pair of atoms forms the most polar bond? Bonding between a metal and a nonmetal is.
SOLVED Which pair of atoms forms the most polar bond? N and F and F N and O Submit
Web polar bonds are intermediate between pure covalent bonds and ionic bonds. In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge between them. In other words, a polar bond forms an electric dipole. Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most.
Answered Which of the following atom pairs form… bartleby
Web polar bonds are intermediate between pure covalent bonds and ionic bonds. Web bonds between two nonmetals are generally covalent; Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most polar: An oxidant is a substance that can accept the electrons from another reagent. Web in a polar bond, one atom.
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts
Web a polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the. Web in a polar bond, one atom has a partial positive electrical charge, while the other atom has a partial negative electrical charge. Web bonds between two nonmetals are generally covalent; Web polar bonds are intermediate between pure.
PPT Chemistry 103 PowerPoint Presentation, free download ID6580778
Web polar bonds are intermediate between pure covalent bonds and ionic bonds. In other words, a polar bond forms an electric dipole. Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most polar: In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge between.
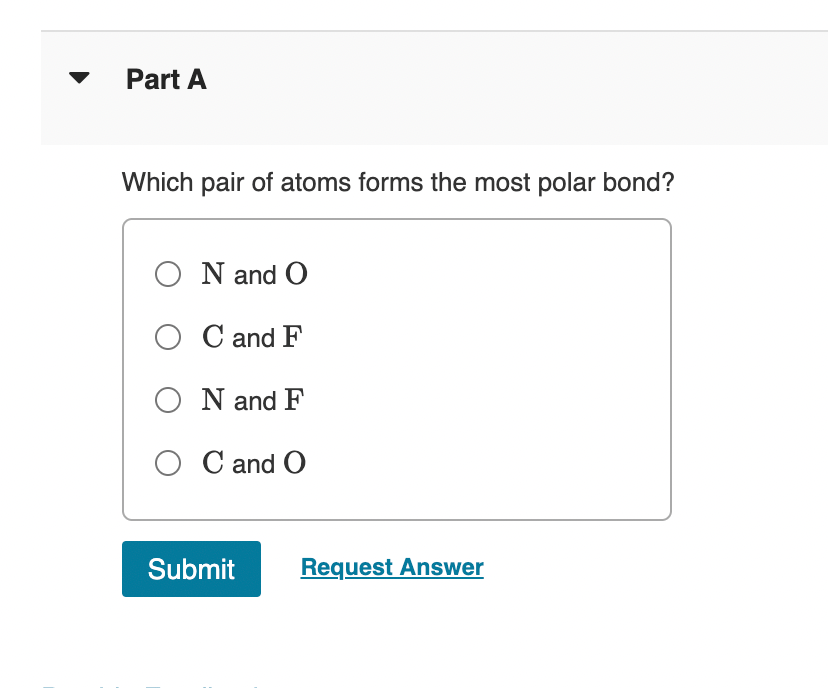
Solved Part A Which pair of atoms forms the most polar bond?
Bonding between a metal and a nonmetal is often ionic. Web a polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the. Web polar bonds are intermediate between pure covalent bonds and ionic bonds. In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative.
Solved Part A Which pair of atoms forms the most polar bond?
An oxidant is a substance that can accept the electrons from another reagent. Web a polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the. In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge between them. Web bonds between two nonmetals are.
Solved Which pair of atoms forms the most polar bond? N and
In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge between them. Web polar bonds are intermediate between pure covalent bonds and ionic bonds. Web which pair of atoms forms the most polar bond? An oxidant is a substance that can accept the electrons from another reagent. Web bonds between two nonmetals are.
[ANSWERED] Which pair of atoms forms the most polar Physical Chemistry
Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most polar: Web bonds between two nonmetals are generally covalent; In other words, a polar bond forms an electric dipole. Web polar bonds are intermediate between pure covalent bonds and ionic bonds. Bonding between a metal and a nonmetal is often ionic.
In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge between them. Web which pair of atoms forms the most polar bond? An oxidant is a substance that can accept the electrons from another reagent. Bonding between a metal and a nonmetal is often ionic. Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most polar: In other words, a polar bond forms an electric dipole. Web bonds between two nonmetals are generally covalent; Web in a polar bond, one atom has a partial positive electrical charge, while the other atom has a partial negative electrical charge. Web a polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the. Web polar bonds are intermediate between pure covalent bonds and ionic bonds.
Bonding Between A Metal And A Nonmetal Is Often Ionic.
In a nonpolar bond, atoms share electrons equally so there is no partial positive or negative charge between them. An oxidant is a substance that can accept the electrons from another reagent. Web a polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the. Web polar bonds are intermediate between pure covalent bonds and ionic bonds.
Web Which Pair Of Atoms Forms The Most Polar Bond?
Web in a polar bond, one atom has a partial positive electrical charge, while the other atom has a partial negative electrical charge. In other words, a polar bond forms an electric dipole. Web bonds between two nonmetals are generally covalent; Web use the electronegativity values shown in figure 2.3 to rank the following bonds from least polar to most polar:









![[ANSWERED] Which pair of atoms forms the most polar Physical Chemistry](https://i2.wp.com/media.kunduz.com/media/sug-question-candidate/20220616130601014530-4627221.jpg?h=512)